Low tortuosity electrode design facilitates the development of high-performance LMO batteries
A new study, published on Dec. 21 in Energy Material Advances, provides a strategy to enhance dynamic phase stability and suppress Mn dissolution at the mesoscopic scale, promoting the development of high-performance spinel LiMn2O4 (LMO) electrodes.
"The development of electric vehicles and electric bicycles has sparked interest in powered lithium-ion batteries," said paper author Jia Xie, professor at school of Electrical and Electronic Engineering, Huazhong University of Science and Technology (HUST). "It is critical to developing advanced electrode materials with excellent rate performance, cycling stability and high-energy densities."
Xie explained that LMO is deemed as a promising cathode material in power lithium-ion batteries, owing to its low cost, reliable security, high operating voltage and fast 3D lithium-ion transport channels.
But the cycling stability of LMO limits its large-scale application. According to Xie, the challenges are the irreversible phase transition and Mn dissolution. The irreversible phase transition from spinel-structured LiMn2O4 to defect spinel-structured λ-MnO2, LiMn3O4 or even rock-salt-structured MnO occurs during repeated cycling.
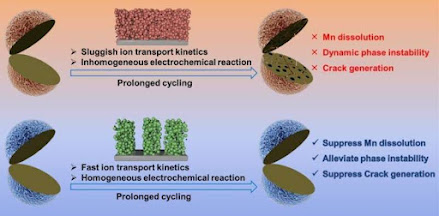
Continuous accumulation of irreversible phase transitions leads to crack generation in LMO particles. The newly exposed surface created by cracks of LMO particles can interact with the electrolyte and thus promote the dissolution of Mn from LMO, resulting in an over-lithiated LMO surface, which in turn accelerates irreversible phase transition and particle cracks. All of these contribute to the deterioration of LMO particles and fast performance decay, especially under fast charging conditions.
According to Xie, advanced strategies including element doping, surface modification and morphological regulation have been proposed to alleviate the degradation of LMO particles. For instance, metal elements (Mg, Ni) and non-metal elements (B, P) are employed as doping elements to replace trace amounts of Mn, forming stable chemical bonds among doping elements and O. Robust chemical bonds keep LMO in a stable structure, resulting in better electrochemical performance than the pristine LMO.
Al2O3, TiO2, ZrO2 and Li3PO4 and other compounds are used as coating layers to cover the LMO surface. The coating layer is equivalent to an artificial CEI, which reduces the direct contact between LMO and the electrolyte, thereby suppressing the dissolution of Mn and volume change. In addition, regulating the morphology of LMO, such as nanostructured materials and truncated octahedral crystal structure design, is also an effective means to improve the performance. These strategies focus mainly on the modification of LMO material itself.
"This paper mitigates the degradation of LMO from the perspective of electrode structure design, and thus improves the cycling stability of LMO," Xie said. "The ice-templating method was adopted to construct low-tortuosity LMO electrode based on the CMC binder. The [resulting] electrodes have straight channels through the electrode in the cross section."
"The low-tortuosity structure enables LMO electrodes' fast lithium-ion diffusion and small concentration polarization, leading to uniform electrochemical reaction within micro-regions of the electrodes. Furthermore, the fast lithium-ion transport kinetics and even mesoscopic-scale reactions of low-tortuosity LMO electrode effectively alleviate irreversible phase transition and Mn dissolution as well as suppress cracks generation in LMO particles," Xie said.
"The fast ion transport kinetic behavior and stable phase structure give the low tortuosity LMO electrode excellent rate performance and cycling stability, which makes it a more competitive cathode," Xie said. "Low tortuosity electrode structure design provides a new way to develop high-performance LMO batteries."
For more such news & interesting articles or how can it affect in your life subscribe to our newsletter


Comments
Post a Comment